Which Statement Correctly Describes the Electrons in a Water Molecule
10 Which statement best describes why water is called a polar molecule. Which statement best describes why a water molecule is polar.

Hydrogen Bonds In Water Article Khan Academy
Carbon dioxide is an example of a molecule where the component atoms of carbon and oxygen are held together by chemical bonds.
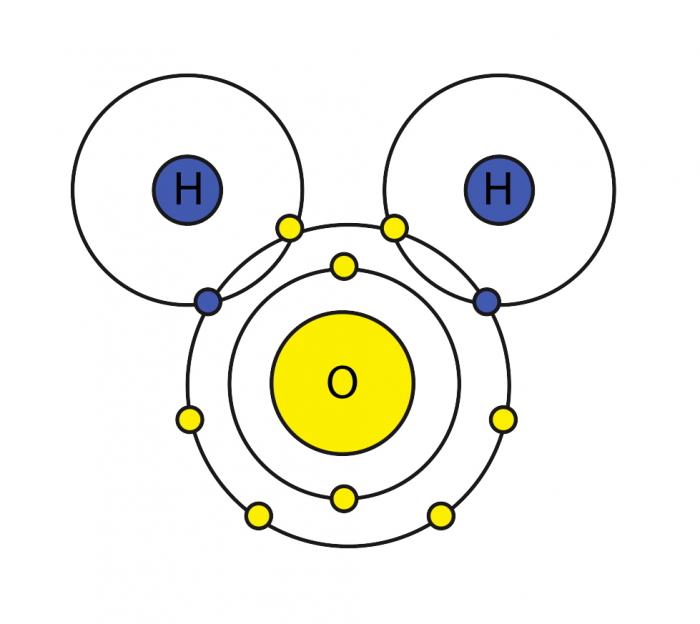
. An _____ is made up. The hydrogen atoms have a partial positive charge and are attracted to the oxygen atom in another molecule which has a partial negative charge. Electrons are always shared equally.
7 Why is water not a universal solvent. Electrons are pulled closer to the oxygen atom. 9 Why is water important to living things quizlet.
Oxygen can only form one single bond with another atom share two electrons. Oxygen exerts a stronger pull on the shared electrons than the hydrogens do. O There are two hydrogen atoms but only one oxygen atom.
Up to 256 cash back Which statement correctly describes the electrons in a water molecule. Hydrogen is highly reactive D. Which statement best describes why a water molecule is polar.
This imparts partial negative charge to the oxygen atom and partial positive charge to hydrogen atoms. Electrons are always shared equally. The hydrogen bonding between water molecules allows water to be cohesive and adhesive.
A water molecule consists of two atoms of hydrogen and two atoms of oxygen. Water cannot dissolve oilsB. The combination of chemical energy stored in each atom makes the bonds strong.
Which statement correctly describes the behavior of valence electrons in this bond. Which statement is true about the following molecule. Electrons are an equal distance between hydrogen and oxygen.
The strong nuclear energy bonds the atoms together. O Oxygen is bigger than hydrogen ging it a stronger positive charge. 11 Which statement best describes why water is called a polar molecule.
Hydrogen is more electronegative than oxygen causing a partial negative charge on hydrogen and a partial charge on oxygen. 6 Which statement best explains why the study of water is relevant to the study of living organisms. Which statement correctly describes the reason for these chemical bonds1 point A.
1 pointThe molecule is composed of four atomsThe molecule contains oxygen atomsThe molecule is waterThe molecule represents an element. 7 What are 3 reasons why water is important. Water is cohesive sticks to itself and adhesive sticks to other substances.
Chemistry questions and answers. An element from the sodium is shared with the fluorine atom forming fluoride iron. An atom has 3 protons 4 neutrons and 3 electrons.
What is the shape of the molecule located to the right. The oxygen atom acts as if it has a positive charge while the hydrogen atoms act as if they. 8 Why is the water essential for life class 9.
Oxygen can form one double bond with another atom share four electrons. Since oxygen is more electronegative as compared to hydrogen atoms the shared electrons are attracted towards the oxygen atom. 12 Which statement best describes why water is a polar molecule.
Water is a an ionic crystalline compound. A water molecule consists of one oxygen atom bonded to two hydrogen atoms by covalent bonds. Oxygen is bigger than hydrogen giving it a stronger positive charge.
Which statement best describes why a water molecule has polar properties. Oxygen exerts a stronger pull on the shared electrons than the hydrogens do. Electrons are always shared equally.
The shape of water molecule is asymmetrical C. All the atoms act as if they have a negative charge. A water molecule is composed of two atoms.
A sample of water has a definite volume and a definite shape. Which statement correctly describes the bonding between water molecules. All the atoms act as if they have a positive charge.
Electrons are located inside the nucleus of the hydrogen atoms. Which statement below correctly describes water. Which of the statements below correctly describes the partial charges on the atoms in a water molecule.
8 Which statement best explains why water molecules are polar. Unequal sharing of electrons. 10 What are the properties of water that make it essential to life.
The atoms in a water molecule are bonded covalently. 9 Which statement best describes why water is a polar molecule.
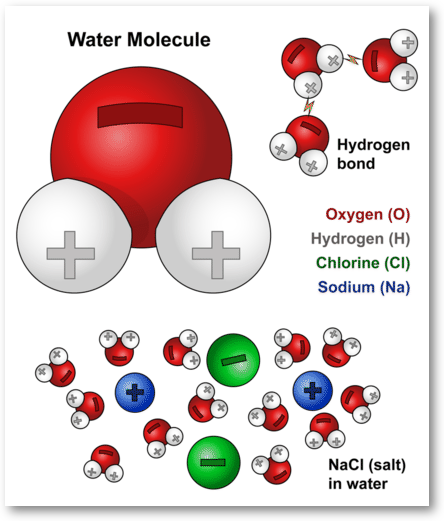
Water Molecules And Their Interaction With Salt U S Geological Survey

The Configuration Of The Water Molecule Earth 111 Water Science And Society

Comments
Post a Comment